The BCOP (Bovine Corneal Opacity and Permeability) assay is an in vitro eye irritation test method developed by Gautheron et al. (1992), which uses living bovine corneal tissue, obtained as a by-product from abattoirs, to evaluate the potential ocular irritancy of a test article. Types of injury caused by exposure to the test article are quantitatively measured by changes in opacity and permeability to fluorescein.
The BCOP assay allows for the investigation of the mechanism of the damage caused. Corneal opacity can be caused by protein denaturation or the induction of stromal swelling, while corneal permeability reflects a loss in corneal barrier function and cell-to-cell membrane junctions of the corneal epithelium.
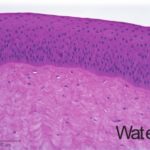
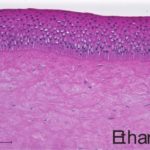
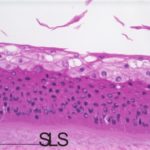
An additional histological endpoint can be added to assess the corneal swelling, hydration, or morphological alterations in the cornea. This assessment evaluates the type of observed lesions and the depth of injury into the corneas.